The United States Pharmacopeia (USP) Convention’s Chapter 800, “Hazardous Drugs—Handling in Healthcare Settings,” is set to become effective December 1, 2019. The new standards provide detailed specifications for the handling of hazardous drugs in order to protect pharmacy staff from health risks associated with harmful chemicals. Hazardous drugs, such as antineoplastic drugs used in chemotherapy, are identified by the National Institute for Occupational Safety and Health (NIOSH) based upon criteria that includes carcinogenicity and toxicity. Pharmacies that handle these hazardous drugs are primarily regulated by the State Boards of Pharmacy, and may also be inspected by the U.S. Food & Drug Administration.
As a result of the imminent USP 800 chapter, many pharmacies in hospitals and cancer clinic settings require substantial modification to comply with the safety requirements. The authoritative new standards address the sequence and types of spaces required; appropriate finishes for floors, counters, and ceilings; necessary components such as a sink and emergency eyewash station; and environmental controls, including negative pressure spaces for unpacking and compounding the drugs. In many cases, pharmacies will need to expand or reconfigure their space in order to accommodate the prescribed spaces.
Creating a Safe Process: Unpack, Prepare, and Store
Pharmacists and hospital facility administrators must focus on three primary functions in the handling of hazardous drugs in order to comply with USP 800: unpacking, preparation (compounding), and storage.
First, the new rules call for hazardous drugs to be unpacked in a negatively pressured or neutral environment to mitigate contact with the residues and gasses of harmful chemicals in the containers. Pharmacy staff will no longer be able to unpack the drugs in general pharmacy spaces, typically designed with positive pressurization. This will require the creation of a separate, negatively pressured drug storage room.

Pharmacies may need to renovate their facilities in order to accommodate new USP 800 standards.
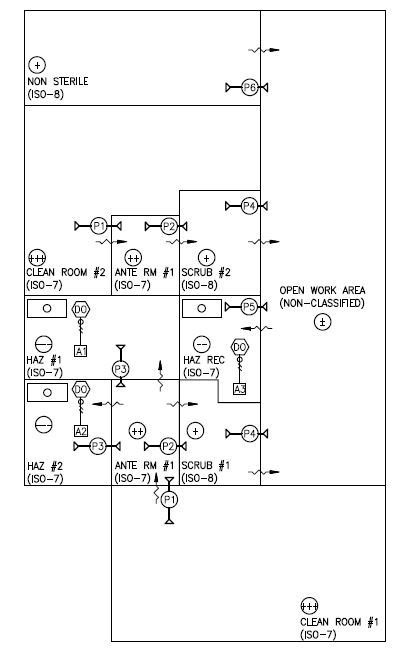
Pressurization and ISO requirements for typical USP 800 pharmacy.
Second, USP 800 requires that hazardous drug compounding take place in a type of containment primary engineering control (C-PEC) called a Biological Safety Cabinet, providing a cleanroom environment of ISO Class 5. The (C-PEC) must run continuously, with emergency power available. The C-PEC must be located in a secondary engineering control (C-SEC) room. The C-SEC (compounding room) must be maintained at negative pressure, be 100 percent exhausted to the outside, have HEPA filtered supply grilles, and have a minimum of 30 air changes per hour (ACH). The room must be at a cleanroom environment of ISO Class 7, and have a negative pressure of 0.02 inches water relative to all adjacent spaces. This pressure must be continuously maintained and monitored, even when unoccupied.
.jpg?sfvrsn=2de6b128_0)
Compounding hazardous drugs must take place in a biological safety cabinet (C-PEC).
The C-SEC must have low exhaust grilles, including exhaust grilles behind refrigerators. Exhaust fans must be located on the roof with a high-velocity vertical discharge stack. The stack must be labeled. The exhaust fans and HVAC system must be on emergency power. Sprinkler heads must be flush-mounted and concealed for clean room use, and lights must be sealed, cleanroom-style fixtures.
Floors must feature seamless, sheet-style vinyl flooring, often specified as anti-fatigue, rubber sheet flooring bonded to rubber backing, which improves ergonomics. The flooring is smooth and impervious, with sealed seams, and is resistant to disinfectant agents and easy to clean. Wall protection features, including corner guards and wall guards, help protect against wear and tear from carts and hand trucks. Counters must also be made of impervious, non-porous materials with integral sinks for durability and to maximize infection controls. Other requirements for the space include an eyewash station. Modular casework storage systems should be specified for flexibility and should be integrated during the space planning process.
Temperature and humidity requirements for the C-SEC room are lower than in the past. These requirements are addressed in USP 797, with revisions that call for a standard of a continuously maintained temperature of 20 degrees C (68 degrees F) and 60 percent humidity. This condition can be met with traditional HVAC equipment, but because this is an absolute maximum, many pharmacy consultants are recommending the compounding room be kept at lower conditions to allow some buffer in the temperature to avoid having to shut down operations in the case of a higher fluctuation. For example, some pharmacies are opting for a temperature of 66 degrees F and 50 percent humidity. This requires the air to the supply air to be cooled below 47 F (dewpoint) for sufficient dehumidification, usually necessitating special HVAC equipment capable of reaching this low dewpoint. Where possible, if the pharmacist agrees to maintain 67 F/50% as a minimum, standard chilled water systems can be used. Protocols are required to address allowable time and level of temperature and humidity excursions along with measures to be taken.
Entry to the C-SEC compounding room must be through an anteroom. The air-locked anteroom must be maintained at 0.02 inches positive pressure relative to the hazardous compounding and 0.01 inches positive to other spaces. This space must also be ISO Class 7 and have HEPA filtered supply grilles. The anteroom must be equipped with a sink for handwashing.
Third, USP 800 calls for separate storage of hazardous drugs in an externally ventilated, negative pressure room, with a minimum of 12 ACH. This may require the conversion of an existing space into a storage area that meets the USP requirements.
Pharmacy Automation: Dispensing Robots and Conveyor Systems
Dispensing robots and conveyor systems now play a critical role in the reduction of errors; staff efficiency; and how drugs are stored, filled, and delivered. Robots enable technicians and pharmacists to spend less time manually finding prescriptions and more time with patients. Although not suitable for compounding drugs, these intelligent systems free up space and streamline operations through efficient retrieval and dispensation, and by storing drugs within the robot rather than on static shelves. While retrofitting pharmacy spaces to meet USP 800 requirements, pharmacists and facility administrators may want to explore options to incorporate this technology.
Other technology measures that must be addressed include security, an important concern in the protection of controlled substances. Security gates, cameras, card readers, access-controlled cabinets, and staff lockers with glass fronts are all part of necessary pharmacy security measures.

Automation can enhance efficiency but may require reconfiguration of spaces.
Resources Regarding the New Standards
While many hospital pharmacists are aware of the pending new standards, facility managers and administrators may be interested in learning more about the implications as they apply to the physical space. The American Society of Health System Pharmacists (ASHP) is one important source, with a number of articles posted to update hospital and clinic staff on the regulations. More information can also be found on the USP website under “Compounding Standards.” These new standards are an important step forward in protecting the health and safety of pharmacy staff as they unpack, prepare, and store hazardous drugs.